Spdf Orbitals. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. Spdf orbitals are energy sub level or sub shells. At the first energy level, the only orbital available to electrons is the. N represents the energy level. The letters and words refer to the visual impression left by the fine. These orbitals have different shapes (e.g. Helium, has two protons and two neutrons that comprise the. This video explains s, p, d, and f orbitals, sublevels, and their shapes. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. It discusses the 4 quantum numbers n, l, ml, and ms. For k shell there is only one s subshell. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. The orbitals are of 4 types.
Spdf Orbitals , Watch These Videos To Learn More And Ace Your Prelim Chemistry Exam!
What Is The Shape Of F Orbital Example. At the first energy level, the only orbital available to electrons is the. These orbitals have different shapes (e.g. N represents the energy level. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. It discusses the 4 quantum numbers n, l, ml, and ms. Spdf orbitals are energy sub level or sub shells. Helium, has two protons and two neutrons that comprise the. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. The letters and words refer to the visual impression left by the fine. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. The orbitals are of 4 types. This video explains s, p, d, and f orbitals, sublevels, and their shapes. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. For k shell there is only one s subshell. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals.
This number divides the shells into subshells (also called sublevels or orbitals).
N represents the energy level. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. Recently in my chemistry classes, the teacher spoke about spdf configuration and then said that we'll be taught about it in higher classes. A wide variety of d electron orbitals options are. Other orbitals include the p, d and f orbitals. Struggling with electronic configuration and spdf notation in prelim chemistry? It by spdf configuration, he meant orbital configuration. The letters and words refer to the visual impression left by the fine. Helium, has two protons and two neutrons that comprise the. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. We need you to answer this question! So far, we've seen that we can explain some experimentally what we need to do is use different (linear) combinations of the spdf domains to create new domains. .p orbitals, d orbitals and f orbitals ñ the 'spaces' in which an electron occupied quantum level spdf notation for 53 iodine, i ?, spdf notation for 54 xenon, xe ?, spdf notation for 55 caesium, cs. In chemistry, spdf are the names of the orbitals. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. Find out information about spdf. Normal basis set atomic orbitals. For k shell there is only one s subshell. For historical reasons the designated letters stand for sharp, principal, diffuse, and fundamental, based on the spectral line observations associated with. At the first energy level, the only orbital available to electrons is the. It discusses the 4 quantum numbers n, l, ml, and ms. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. The orbital arrangement of an atom's electrons. N represents the energy level. From wikimedia commons, the free media repository. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with. Watch these videos to learn more and ace your prelim chemistry exam! These orbitals have different shapes (e.g. This number divides the shells into subshells (also called sublevels or orbitals). The s orbitals are spherical, while p orbitals are polar and oriented in particular directions (x, y, and z). Can anyone explain to me in laymans terms the whole s p d f business?
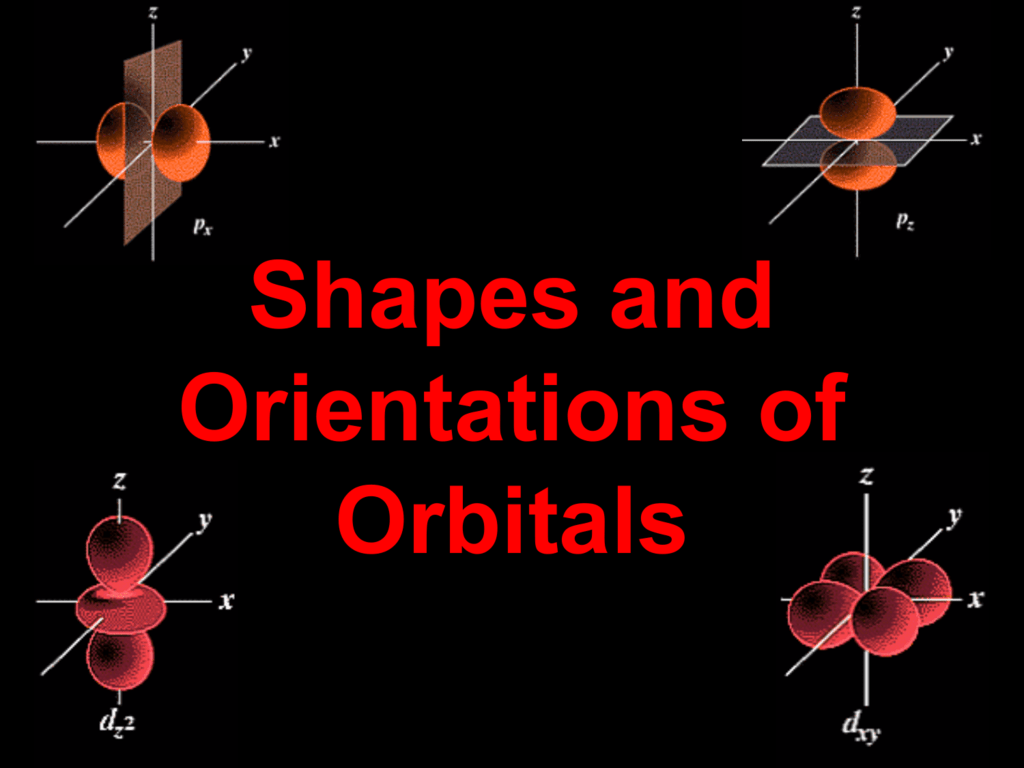
Molecular Structure Atomic Orbitals : The S Orbitals Are Spherical, While P Orbitals Are Polar And Oriented In Particular Directions (X, Y, And Z).
Ninth Grade Lesson Introduction To Electron Orbital Levels. N represents the energy level. The orbitals are of 4 types. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. Helium, has two protons and two neutrons that comprise the. This video explains s, p, d, and f orbitals, sublevels, and their shapes. It discusses the 4 quantum numbers n, l, ml, and ms. Spdf orbitals are energy sub level or sub shells. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. The letters and words refer to the visual impression left by the fine. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. At the first energy level, the only orbital available to electrons is the. For k shell there is only one s subshell. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. These orbitals have different shapes (e.g.
S P D F Orbitals Explained 4 Quantum Numbers Electron Configuration Orbital Diagrams Youtube Electron Configuration Chemistry Worksheets Quantum . But I'm Sorta Curious To Know That What Is Spdf Configuration And.
Diagram Construct The Orbital Diagram For F Sapling Full Version Hd Quality F Sapling Rpmtransmissions Causses En Ailes Fr. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. The orbitals are of 4 types. These orbitals have different shapes (e.g. At the first energy level, the only orbital available to electrons is the. Spdf orbitals are energy sub level or sub shells. It discusses the 4 quantum numbers n, l, ml, and ms. This video explains s, p, d, and f orbitals, sublevels, and their shapes. For k shell there is only one s subshell. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively.
Shutterstock Puzzlepix . In chemistry, spdf are the names of the orbitals.
Parsing Spdf Orbital Hybridization And Simple Bonding. Spdf orbitals are energy sub level or sub shells. The letters and words refer to the visual impression left by the fine. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. This video explains s, p, d, and f orbitals, sublevels, and their shapes. For k shell there is only one s subshell. It discusses the 4 quantum numbers n, l, ml, and ms. These orbitals have different shapes (e.g. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. Helium, has two protons and two neutrons that comprise the. N represents the energy level. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. At the first energy level, the only orbital available to electrons is the. The orbitals are of 4 types.
Ks9027 Set Of Seven F Orbital Models Klinger Educational Products , 821 D Electron Orbitals Products Are Offered For Sale By Suppliers On Alibaba.com, Of Which Hydraulic Parts Accounts For 1%, Car Polishers Accounts For 1%.
F Orbital Shape Ewt. These orbitals have different shapes (e.g. It discusses the 4 quantum numbers n, l, ml, and ms. For k shell there is only one s subshell. Helium, has two protons and two neutrons that comprise the. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. The letters and words refer to the visual impression left by the fine. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. Spdf orbitals are energy sub level or sub shells. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. This video explains s, p, d, and f orbitals, sublevels, and their shapes. N represents the energy level. At the first energy level, the only orbital available to electrons is the. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. The orbitals are of 4 types.
Visualizing Electron Orbitals : S Orbitals Have 2 Vectors P Orbitals Have 6 Vectors D Orbitals Have 14 Vectors F Orbitals Have 14 Orbitals And Nuclear Shape.
Shapes Of Orbitals And Sublevels. Helium, has two protons and two neutrons that comprise the. Spdf orbitals are energy sub level or sub shells. These orbitals have different shapes (e.g. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. The letters and words refer to the visual impression left by the fine. This video explains s, p, d, and f orbitals, sublevels, and their shapes. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. The orbitals are of 4 types. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. At the first energy level, the only orbital available to electrons is the. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. N represents the energy level. For k shell there is only one s subshell. It discusses the 4 quantum numbers n, l, ml, and ms. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively.
Bilangan Kuantum Elektron Atom Ekimia Web Id - When The Spdf Orbitals Are Subjected To A 3D, Visual Summing, They Present Anything But A Neat Assemblage Of The Electron Spaces Needed To Handle The Required Number Of Periodic Electrons.
S P D F Orbitals And Angular Momentum Quantum Numbers. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. At the first energy level, the only orbital available to electrons is the. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. The letters and words refer to the visual impression left by the fine. Spdf orbitals are energy sub level or sub shells. This video explains s, p, d, and f orbitals, sublevels, and their shapes. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. It discusses the 4 quantum numbers n, l, ml, and ms. These orbitals have different shapes (e.g. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. Helium, has two protons and two neutrons that comprise the. For k shell there is only one s subshell. N represents the energy level. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. The orbitals are of 4 types.
The Shape Of Orbitals Quantum Numbers : But I'm Sorta Curious To Know That What Is Spdf Configuration And.
Bohr Model S P D F Orbitals Flashcards Quizlet. For k shell there is only one s subshell. The letters and words refer to the visual impression left by the fine. This video explains s, p, d, and f orbitals, sublevels, and their shapes. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. At the first energy level, the only orbital available to electrons is the. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. Spdf orbitals are energy sub level or sub shells. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. N represents the energy level. Helium, has two protons and two neutrons that comprise the. The orbitals are of 4 types. It discusses the 4 quantum numbers n, l, ml, and ms. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. These orbitals have different shapes (e.g.
Parsing The Spdf Electron Orbital Model Chemistry Education Chemistry Classroom Chemistry Lessons : This Number Divides The Shells Into Subshells (Also Called Sublevels Or Orbitals).
Atomic Orbitals Definition Shapes Examples And Diagrams. It discusses the 4 quantum numbers n, l, ml, and ms. At the first energy level, the only orbital available to electrons is the. The orbitals are of 4 types. Helium, has two protons and two neutrons that comprise the. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. Spdf orbitals are energy sub level or sub shells. The letters and words refer to the visual impression left by the fine. These orbitals have different shapes (e.g. N represents the energy level. For k shell there is only one s subshell. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. This video explains s, p, d, and f orbitals, sublevels, and their shapes.
Q Is It Possible For An Atomic Orbital To Exist Beyond The S P F And D Orbitals They Taught About In School Like Could There Be A Other Letter Orbital Beyond , .P Orbitals, D Orbitals And F Orbitals Ñ The 'Spaces' In Which An Electron Occupied Quantum Level Spdf Notation For 53 Iodine, I ?, Spdf Notation For 54 Xenon, Xe ?, Spdf Notation For 55 Caesium, Cs.
Electronic Structure And Periodic Table Mcat Review. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. These orbitals have different shapes (e.g. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. This video explains s, p, d, and f orbitals, sublevels, and their shapes. For k shell there is only one s subshell. Helium, has two protons and two neutrons that comprise the. Spdf orbitals are energy sub level or sub shells. At the first energy level, the only orbital available to electrons is the. It discusses the 4 quantum numbers n, l, ml, and ms. N represents the energy level. The letters and words refer to the visual impression left by the fine. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. The orbitals are of 4 types.
Orbitals Chemistry For Non Majors . N Represents The Energy Level.
Classification Of Elements Into S P D F Blocks In The Periodic Table. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. N represents the energy level. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. The orbitals are of 4 types. Spdf orbitals are energy sub level or sub shells. These orbitals have different shapes (e.g. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. It discusses the 4 quantum numbers n, l, ml, and ms. This video explains s, p, d, and f orbitals, sublevels, and their shapes. At the first energy level, the only orbital available to electrons is the. For k shell there is only one s subshell. Helium, has two protons and two neutrons that comprise the. The letters and words refer to the visual impression left by the fine.